What does the life science industry in the U.S. look like now? How will it look in the future? What are the factors that will shape the future? Deloitte has addressed each of these questions in their report “2015 Life Sciences Outlook United States.” This blog post summarizes that report.
What does our U.S. life sciences market look like now?
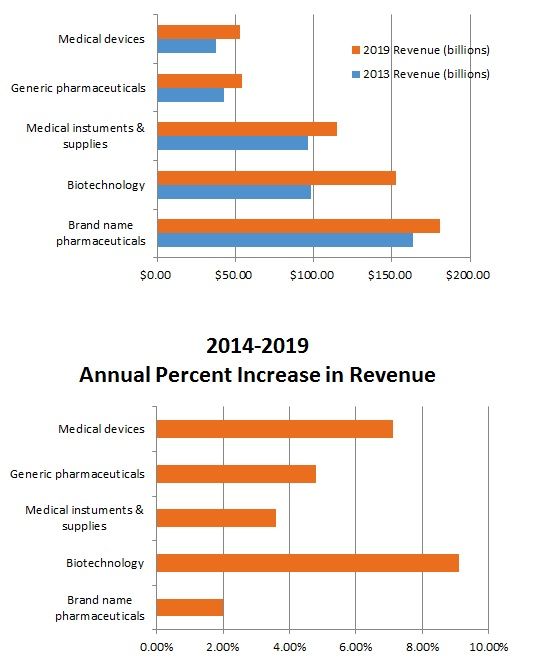
Brand name pharmaceuticals represents the largest segment of the life sciences market, followed by (in order of size) biotechnology, medical instruments and supplies, generic pharmaceuticals, with medical devices being the smallest segment. The two segments expected to have the most significant growth over the next several years are biotechnology (anticipated annual growth of 9.1% from 2014 2019) and medical devices (anticipated annual growth of 7.1% from 2014 2019). See charts below.
Key issues for the U.S. life sciences market:
Deloitte identified six key issues that life sciences companies will need to grapple with:
- Market reconfiguration and consolidation
- Pricing pressures
- Health reform and shift to value
- Research and development productivity
- Disruptive technologies
- Risk, regulation and compliance
We’ll summarize a few of the pressures and developments in each of these issue categories.
Market reconfiguration and consolidation:
In the U.S., pressures such as expiring patents, growth in new markets and value-based reimbursements are triggering companies to reconfigure their business models as they search for the right scale and right product mix for today’s competitive environment. These pressures resulted in significant merger and acquisition (M&A) activity, especially in the biopharma and medtech areas. Emerging markets are expected to fuel this trend going forward. M&A activity among health care providers and payers will also affect buying decisions and a shift from business-to-consumer sales to business-to-business negotiations with institutional decision makers.
Pricing pressures:
Everyone wants to pay less. The Accountable Care Act (ACA) reforms are resulting in increased pricing pressures. Under the ACA, generic versions of off-patent biotech drugs and biosimilars were granted a shortened regulatory approval pathway and medical devices became subject to a 2.3 percent excise tax. Health plans are narrowing their drug formularies and creating tiered co-payment plans requiring greater out-of-pocket investment by the consumer to have access to brand-name drugs. And hospital systems are negotiating hard for favorable pricing.
Health reform and shift to value:
This is a sea change: The ACA has engineered a shift from volume-based to value-based care which will require that life sciences companies to demonstrate not only a product’s clinical efficacy and safety, but also the economic value in comparison with the next-best alternative. Life sciences companies will need to design their clinical trials in such a way that they can make competitive comparisons on such inputs as hospital revenue, quality of care, costs to the health care system, efficacy, side effects and tolerability.
R&D productivity:
A Deloitte and Thomson Reuters study of 12 large, global life sciences companies found that expected return on late-stage pipeline projects has declined in recent years while the cost to develop and launch new medicines has increased substantially. Medtech venture capital investment peaked in 2009 and has been declining steadily since. These pressures may cause companies to explore various ways to collaborate in the sharing of risk, intellectual property, resources, costs – and potential rewards associated with bringing new products and therapies to market.
Disruptive technologies:
Look over your shoulders, guys. Non-traditional players may come to market with disruptive technologies that engage consumers in new ways. The next generation of smart medtech devices may challenge the way care is currently delivered.
Risk, regulation and compliance:
Patient safety and data transparency are receiving intense regulatory scrutiny and enforcement. While FDA review process has traditionally been more demanding and lengthy than that in other countries, regulatory agencies globally are becoming increasingly sophisticated and stringent such that approval in the U.S. will no longer assure elsewhere.
The outlook?
It will be a bumpy ride as the segments of the life sciences market recalibrate their business models, readjust R&D priorities and retool their practices in response to market pressures. Deloitte admonishes that U.S. life sciences companies “should focus on areas in which they excel, improve areas that are important to achieving their goals, and let go of elements that might be holding them back.”
To learn more about how other Life Science companies have partnered with us to overcome road blocks and capacity issues and tapped into our skilled professional talent pool, contact me at 612.703.4236 or email me at: pnorbom@talencio.com. Talencio, LLC has been the preferred provider of vetted, accomplished professionals to the Life Sciences community for nearly eight years.
Sources:
Deloitte, 2015 Life Sciences Outlook United States; Deloitte Touche Tohmatsu Limited, 2014.