Do you feel that your company is being buffeted about in a sea of change? Perhaps that is because it is. By recognizing and planning for the types of changes in our environment that are transforming our present, we will become better able to be in charge of our future. In this blog we’ll discuss five trends from a report recently published by Master Control that are driving change for the medical device industry[i]: the aging global market, growth of emerging markets, consolidation / mergers and acquisitions, consumerism, and increasing regulation. We’ll touch on each of these trends, with special emphasis on increasing regulation. Companies that can capitalize on these trends will become market leaders.
- Aging Global Market
People are living healthier, longer lives. The U.S. Department of Health and Human Services predicts that the percentage of the population aged 60 years or older in developed countries will increase from its present level of 23% to 32% by the year 2050. With the growth of this segment, demand for medical devices will also increase, especially those that focus on diagnosis and prevention.
- Growth of Emerging Markets
Revenue from the emerging markets of China, India and Brazil are expected to drive the medical device industry for the next 50 years, as U.S. device manufacturers pursue a greater share of their sales revenues from those regions. This may necessitate designing in new ways to meet needs in these emerging economies.
- Consolidation / Mergers & Acquisitions
Consolidation, mergers and acquisitions are common corporate strategies for broadening product offerings, reducing competition and withstanding adverse conditions. But increasing company size by acquiring other companies also increases complexity and reduces nimbleness to respond.
- Consumerism
The consumerization of medical devices – and the medicalization of consumer devices – will continue to drive change in the medical device industry. The emergence of personal empowering technologies that leverage specific information to help people live healthier lives is driven in part by smartphones, social media, increased focus on cost and elevated consumer expectations.
- Increased Regulation
In a recent study conducted by the global consulting firm Emergo Group, medical device representatives cited the “changing regulatory environment” as their greatest challenge in 2014, with respondents in all regions indicating that it is more difficult in 2014 than it was in 2013 to register a new device. China was found to be the most challenging region from a regulatory perspective. While Chinese regulators are making strides to facilitate the approval process, a law which took effect in June 2014 also steps up oversight and penalties for overpricing and corporate malpractice.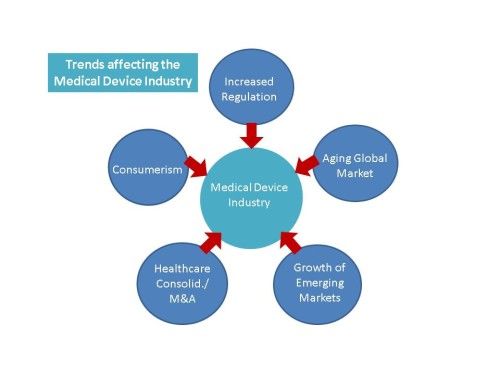
In the U.S, a number of factors are impacting the regulation of medical devices.
Affordable Care Act
The Patient Protection and Affordable Care Act (ACA) of 2010 shifted the philosophy of healthcare to a greater emphasis on higher quality care, as measured by health outcomes. The ACA also imposed a 2.3% tax on medical devices as a way to fund healthcare coverage for previously uninsured Americans. This excise tax has been controversial from the beginning, with opponents of the tax arguing that it will constrain job creation and innovation, and reduce the availability of venture capital for new firms. PricewaterhouseCoopers and the National Venture Capital Association, report that venture funding of U.S. medical device startups was down by 17% in 2013, compared to 2012 funding levels. Another complicating factor relating to the excise tax is the lack of clarity regarding whether mobile health apps and software approved by the FDA for commercialization are subject to this medical device taxation. This tax will continue to be a major thorn in the industry’s side in 2014 as the industry continues its fight for repeal.
FDA’s 510(k) Controversy
Consumer advocacy groups have charged that the FDA’s 510(k) submissions process, the most widely used approval path for medical devices, is outdated and has failed to keep devices which are dangerous and ineffective off the market. In response, in 2010 the FDA proposed 60+ changes to the 510(k) submission policy that will require additional evidence of product safety. But device manufacturers point to the difficulty of getting products to market in the U.S. already, noting that products can be approved in Europe an average of four years ahead of U.S. approval, without increase in recalls or safety issues. The delays prevent U.S. patients from receiving state-of-the-art devices that are available elsewhere, cost devices manufacturers potential sales and contribute to startup companies going out of business while waiting for approval. The struggle to find a balance between regulation and innovation is expected to continue to be a major force impacting the medical device industry in 2014 and beyond.
UDI Legislation
Recent legislation requiring medical device manufacturers to establish a unique device identification (UDI) system for each device (to be phased in over seven years) is another of the FDA’s initiatives to focus on product safety to reduce counterfeiting and increase supply chain security and efficiency.
Are You Prepared?
Each of these trends impacting the medical device industry requires that you be prepared, organized and thorough – and nimble. Companies that are prepared to capitalize on these trends will become market leaders. Are your staff resources appropriately aligned and sufficient to provide your company the competitive advantage in meeting these challenges? Do you have all the right skills in-house?
To learn more about how other life science companies have partnered with us to overcome hurdles and capacity issues, contact us directly at 612.703.4236 or email to: pnorbom@talencio.com. Talencio, LLC is the preferred provider of vetted, accomplished professionals to the Life Sciences community.
*Five Trends Transforming the Medical Device Industry in 2014, Master Control, 2014.